Colesevelam Impurities
Colesevelam Impurities EP USP Related Compounds
Synthink Research Chemicals is a manufacturer and supplier of Colesevelam Impurities which are required for pharmaceutical companies to study quality, stability and biological safety of pharmaceutical products as well as HPLC method validation.
All Colesevelam impurities / related compounds are provided with certificate of analysis (CoA) along with the complete characterization data like 1H-NMR, IR, MASS, HPLC Purity etc. On request, we also provide additional data like 13C-NMR, 13C-DEPT, CHN, TGA, Potency etc (with extra charge/fees). SynThink provides various Colesevelam pharmacopeial and non-pharmacopeial Impurities including – Degradation Impurities, Process Impurities, Potential Impurities, KSM impurities, API impurities etc.
Colesevelam (Welchol) used to lower plasma cholesterol level and used instead of cholestyramine in symptomatic chronic diarrhea (Anticholesteremic Drug). It was developed by GelTex Pharmaceuticals and later acquired by Genzyme.
Please find list of Colesevelam Impurities offered by SynThink :
-
Colesevelam Impurities, Impurities
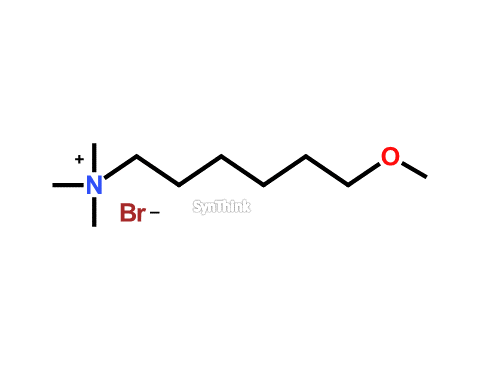
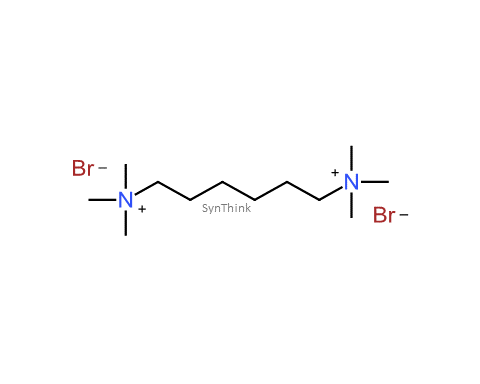
Methoxyquat Bromide
Price : $150 – $790CAS No. : 359436-97-8 Mol F. : C10H24BrNO Mol W. : 254.21 Cat No. : SA10210 In-Stock
-
Colesevelam Impurities, Impurities
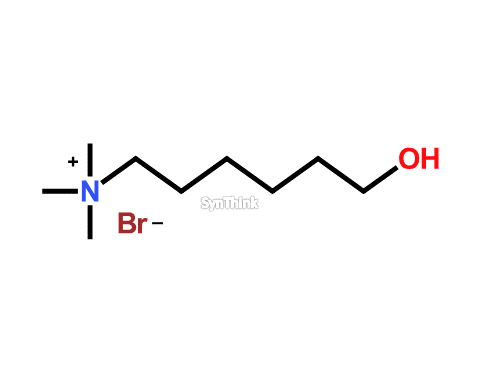
Hydroxyquat
Price : $70 – $250CAS No. : 118843-18-8 Mol F. : C9H22BrNO Mol W. : 240.18 Cat No. : SA10212 In-Stock
-
Colesevelam Impurities, Impurities
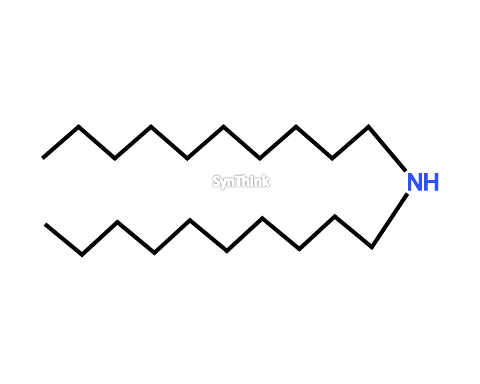
Di-decylamine hydrochloride
Price : $70 – $250CAS No. : 2486-84-2 Mol F. : C20H43N Mol W. : 297.56 Cat No. : SA10206 In-Stock
-
Colesevelam Impurities, Impurities
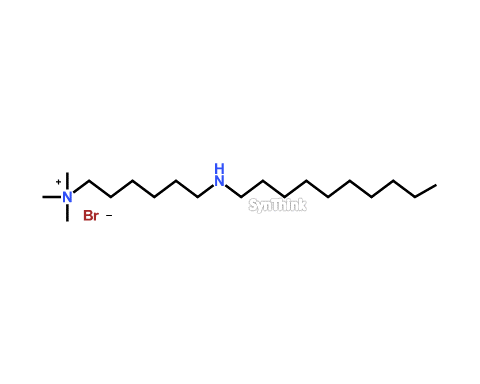
Decylaminoquat
Price : $400 – $1,800CAS No. : NA Mol F. : C19H43BrN2 Mol W. : 379.46 Cat No. : SA10203 In-Stock
-
Colesevelam Impurities, Impurities
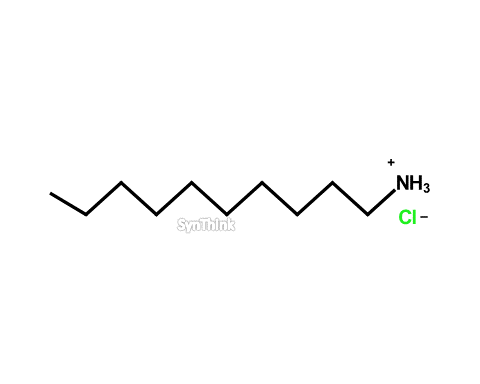
Decylamine hydrochloride
Price : $70 – $250CAS No. : 143-09-9 Mol F. : C10H24NCl Mol W. : 192.75 Cat No. : SA10205 In-Stock
-
Colesevelam Impurities, Impurities
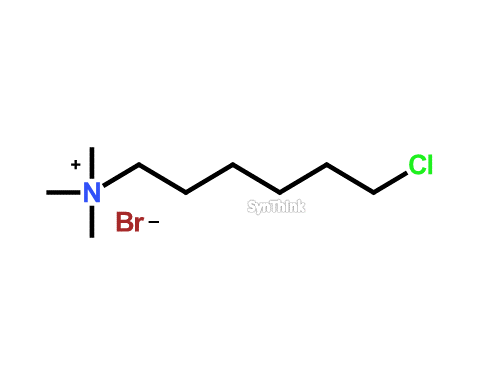
Chloroquat
Price : $70 – $250CAS No. : 93199-84-9 Mol F. : C9H21BrClN Mol W. : 258.63 Cat No. : SA10209 In-Stock
-
Colesevelam Impurities, Impurities
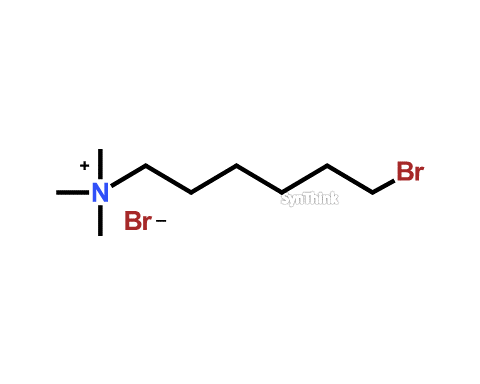
Bromoquat
Price : $70 – $250CAS No. : 32765-81-4 Mol F. : C9H21Br2N Mol W. : 303.08 Cat No. : SA10201 In-Stock
-
Colesevelam Impurities, Impurities
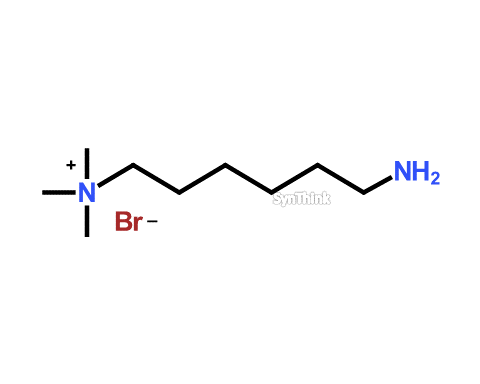
Aminoquat
Price : $70 – $250CAS No. : 33968-67-1 Mol F. : C9H23BrN2 Mol W. : 239.2 Cat No. : SA10202 In-Stock
-
Colesevelam Impurities, Impurities
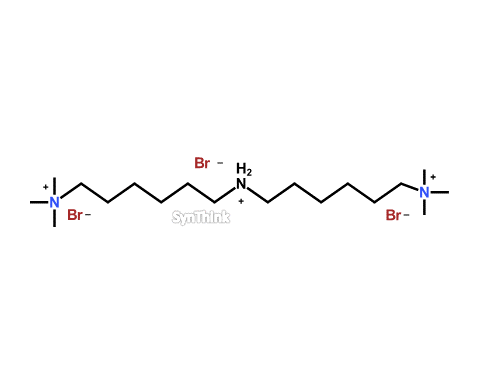
Amino Dihexylquat
Price : $500 – $1,800CAS No. : 2170771-70-5;359436-98-9(FreeBase) Mol F. : C18H43Br3N3– Mol W. : 541.27 Cat No. : SA10204 In-Stock
-
Colesevelam Impurities, Impurities
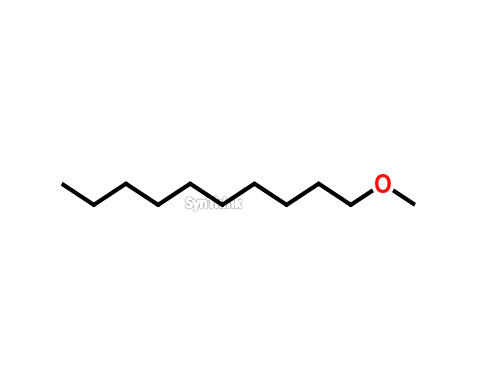
1-Methoxy Decane
Price : $70 – $250CAS No. : 7289-52-3 Mol F. : C11H24O Mol W. : 172.31 Cat No. : SA10207 In-Stock
-
Colesevelam Impurities, Impurities
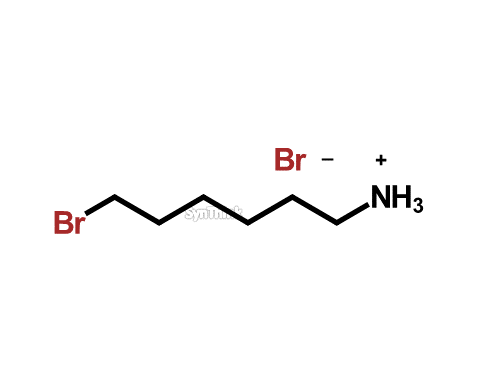
1-Hexanamine 6-bromo hydrobromide
Price : $70 – $250CAS No. : 14502-76-2 Mol F. : C6H14Br2N– Mol W. : 259.99 Cat No. : SA10213 In-Stock
-
Colesevelam Impurities, Impurities
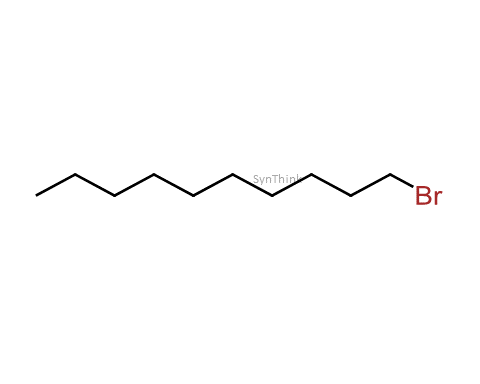
1-Bromodecane
Price : $70 – $250CAS No. : 112-29-8 Mol F. : C10H21Br Mol W. : 221.18 Cat No. : SA10215 In-Stock
-
Colesevelam Impurities, Impurities
Colesevelam Hexamethonium Bromide Impurity
Price : $70 – $250CAS No. : 55-97-0 Mol F. : C12H30Br2N2 Mol W. : 362.19 Cat No. : SA10216 In-Stock
-
Colesevelam Impurities, Impurities
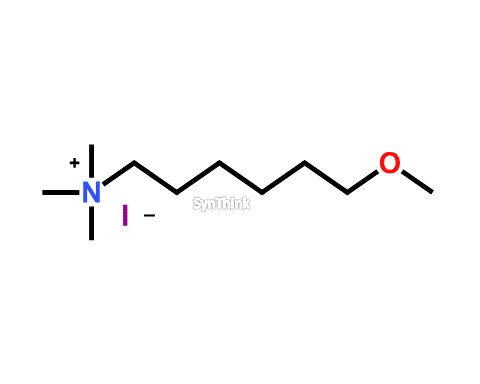
Methoxyquat Iodide
CAS No. : 863031-14-5 Mol F. : C10H24INO Mol W. : 301.21 Cat No. : SA10211 Please Enquire
-
Colesevelam Impurities, Impurities
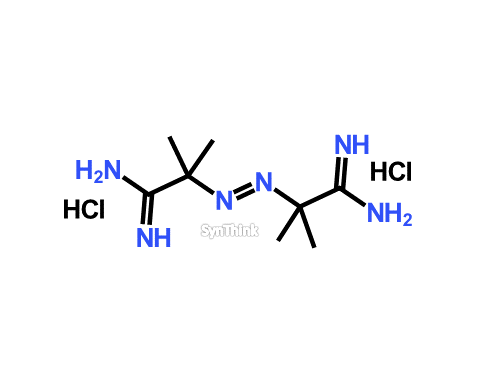
2,2′-azobis(2-amidinopropane) dihydrochloride
Price : $70 – $250CAS No. : 2997-92-4 Mol F. : C8H20Cl2N6 Mol W. : 271.19 Cat No. : SA10208 Limited Stock
Custom Synthesis / manufacturing of Colesevelam Impurities
In cases when required Colesevelam impurities are not available anywhere, they needs to be custom synthesised. In such cases clients need to send the details of the required product to SynThink.
At Synthink, we produce / manufacture / synthesise these impurities using reliable scientific processes / techniques like organic synthesis techniques, degradation and isolation process, enrichment and isolation by chromatography techniques etc.
While doing impurity profiling or any analytical study of a API, factors like potency, retention time (RT), relative retention time (RRT), response factor (RF), relative response factor (RRF) are very important. So, during synthesis / manufacturing process we keep these important factors in mind and produce and supply the highest possible quality material accordingly.
Isolation and purification service is also provided as per clients’ request.
Generally custom synthesis products would be shipped in the in 3-6 weeks (as per difficulty level of the product and time mentioned in quote).
Send us a custom synthesis enquiry or email us – [email protected]
Price and quality of Colesevelam Impurities
Quality of the material is highest priority at Synthink. So, we synthesise / manufacture and supply as pure as possible material to our clients. We always try to provide highest possible quality material to our clients. About price. we always try to give Colesevelam impurities at reasonable price. We can also offer discounts, it depends on particular enquiry and size of order. Please feel free to request discount from us.
Availability, stock and delivery/lead time of the impurities
We are completely aware that impurities in pharma product is always a matter of worry within different departments of a pharmaceutical company. So, at SynThink we are committed to synthesize and characterize required Pharmacopial and non-pharmacopial Reference Standards, API Impurities as well as other working standards in a timely manner.
We keep stock of some products. For various reasons, we can’t keep stock of all products. If the product is in our stock, we can ship / dispatch the product in 48 hrs after successful quality control check. Please feel free to request a quote / stock availability from us.
Characterisation and data integrity
At SynThink, we are aware that Characterisation and data integrity of the impurities is highly important factors to our clients / FDA. We have placed strict internal standard operating procedures. So, our quality control (QA) and quality assurance department check / analyse all data of every impurity batch very carefully as per strict internal standard operating procedures. By doing critical analysis at every stage Synthink is able to provide accurate characterisation and data of every impurity batch. We can also share the electronic copy of CoA and data by email before delivering the material to the client to get confirmation from end user so client won’t get any surprise.
Moreover, on request we can provide characterization or structural elucidation reports of along with impurities
Support: If you need any support or information please contact us or check our support page (https://synthinkchemicals.com/support/)
Keywords: Colesevelam Impurity, Colesevelam Related Compound, Colesevelam Reference Standard, Colesevelam EP Impurity, Colesevelam USP Impurity, Colesevelam Pharmaceutical Standard, Colesevelam impurities for ANDA filing, Analytical standard of Colesevelam , Colesevelam Degradation Impurity, Colesevelam Process Impurity, Colesevelam Potential Impurity, Colesevelam Unknown Impurity, Spiking study, Colesevelam API Impurities, HPLC method validation of Colesevelam , Analytical method development of Colesevelam , impurity profiling of Colesevelam , potency of Colesevelam impurity, retention time (RT) of Colesevelam impurity, relative retention time (RRT), response factor (RF), relative response factor (RRF) of Colesevelam impurity, price of Colesevelam impurity, manufacturer of Colesevelam impurity, supplier of Colesevelam impurity, cost of Colesevelam impurity