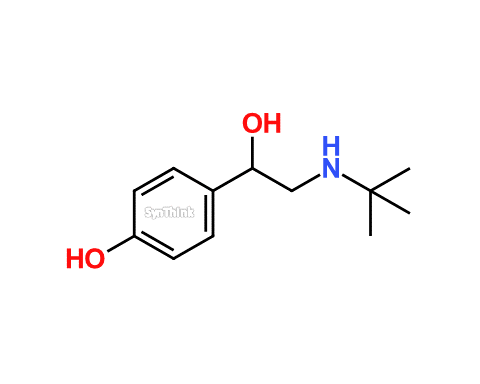
Salbutamol EP Impurity B
| CAS No. | : 96948-64-0 |
| Mol F. | : C12H19NO2 |
| Mol W. | : 209.28 |
| Cat No. | : SA26001 |
- Description
- Additional Info
- Terms and Conditions
- Quick Enquiry / RFQ
Description
| Product Name | Salbutamol EP Impurity B |
| Synonyms | t-Butylnorsynephrine
(1RS)-2-[(1,1-dimethylethyl)amino]-1-(4-hydroxyphenyl)ethanol Salbutamol Deshydroxymethyl Impurity 4-[2-(tert-Butylamino)-1-hydroxyethyl]phenol α-[[(1,1-Dimethylethyl)amino]methyl]-4-hydroxybenzenemethanol |
| Related API | Salbutamol |
| Category | Impurities |
Salbutamol EP Impurity B product with CAS: 96948-64-0 is also known as t-Butylnorsynephrine. This product can be used as a working standard or secondary reference standard. Additional internal validation as per respective FDA regulations/guidelines may require. Salbutamol EP Impurity B is generally used for Quality Control (QC), Quality Assurance (QA) during commercial production of Salbutamol and its related formulations. Moreover, t-Butylnorsynephrine is also used in the process of Abbreviated New Drug Application (ANDA) filing to FDA and toxicity study of respective drug formulation.
If you need different pack size / quantity, please contact us or email us at [email protected]
Certificate of Analysis (CoA) and Characterization Data:
All products are provided with a certificate of analysis (CoA) along with the characterization data like 1H-NMR, MASS, HPLC Purity, MSDS, etc. (whatever relevant and possible). Moreover, additional data like 13C-NMR, 13C-DEPT NMR, GC, GC-MS, IC, Potency, TGA, etc. will be provided on request (extra charges may apply).
This product can be used as a working standard or secondary reference standard. Additional internal validation as per respective FDA regulations/guidelines may require. This product provided by SynThink is generally used for Abbreviated New Drug Application (ANDA) filing to FDA, toxicity study of respective drug formulation, Quality Control (QC) and analytical studies during commercial production of the API]. Please also find out process impurities, degradation impurities, potential impurities of related api/drug developed by our R&D.
Products provide by SynThink are for research and development (R&D) use only
If you need different pack size / quantity, please contact us or email us at [email protected]
Terms and Conditions
In-Stock products will be shipped in 24 Hrs. For others please enquire.
| Shipping | Free for purchase above $5000 |
| Delivery | In-Stock, products will be delivered in 8-15 days via FedEx in the USA, Europe, and other countries. |
| Return Policy | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Order | Place your order online or by email [email protected] (YOU DON'T NEED TO PAY WHILE PLACING THE ORDER. Click here for more info) |
| After Sales Support | Please write to us. Our R&D team will provide all possible support. |