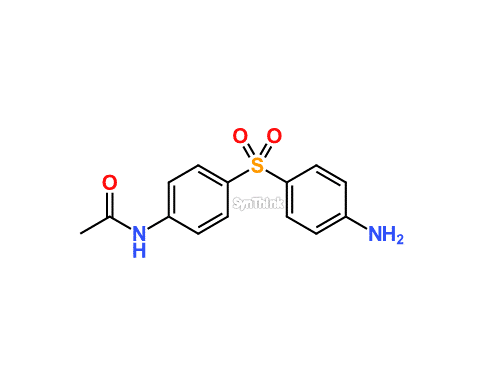
Home / Products / Impurities / Dapsone / Dapsone N-Acetyl Impurity
| Pack Size | Price | Availability | |
|---|---|---|---|
| Please Enquire | Please Enquire |
Enquire about...
Address
Plot No. CP-135, Pimpri-Chinhwad MIDC, Pune-411019, Maharashtra, India.
© 2014-2024 SynThink Research Chemicals. All Rights Reserved.

Register to add the product to RFQ list
Already have an account? Log in here
Don’t have an account? Register here