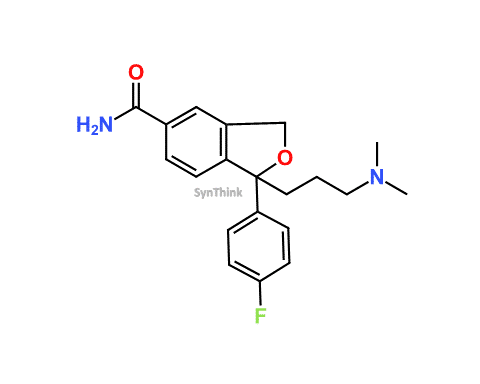
Home / Products / Impurities / Citalopram / Citalopram EP Impurity A; Citalopram Related Compound A
| Pack Size | Price | Availability | |
|---|---|---|---|
| Please Enquire | Please Enquire |
| CoA & Characterization Data | At no extra cost, we provide: 1H-NMR, Mass, HPLC, IR, TGA and Certificate of Analysis (with Purity and Potency.) In rare cases for purity and mass, we need to rely on alternative methods like 1H-NMR, ELSD, GC, GCMS etc. (Applicable for products under ‘Impurities’ category). Additional data will be provided upon request for an additional fee. |
| Shipping & Handling |
Free all over India. Free for orders above $2000 else $100 to rest of the countries In stock products will be shipped in 24 Hrs (after verification of documents).For others please enquire. |
| Delivery Timeline |
India: 2 to 3 business days International: 5 to 8 business days |
| Prices | Prices are on a CPT basis (Incoterms® 2020) and subject to change without notice. |
| Incoterms | CPT (Incoterms® 2020) or as mutually agreed upon |
| Taxes |
For orders within India: GST (extra) For overseas orders: No GST. Any customs duties, taxes, or import charges applicable in the destination country are payable by the customer as per local laws. |
| Return Policy | Returns accepted within 15 days, for valid reasons only. (Check T&C for more info) |
| Order | Please send ‘purchase order’ by email: sales@synthinkchemicals.com (Click here for more info) |
| Payment Terms |
Regular/returning customers: Due 30 days from invoice date New customers: 100% payment in advance |
| After Sales Support | Please write to us. Our R&D team will provide all possible support. |
Enquire about...
Request COA for...
Register to add the product to RFQ list
Already have an account? Log in here
Don’t have an account? Register here