Home / Certified Reference Materials
Certified Reference Materials: Ensuring Accuracy and Reliability in Analytical Testing
Certified Reference Materials (CRMs) play a crucial role in analytical testing across various industries, including the pharmaceutical sector. These materials provide a benchmark for the accurate measurement of substances, enabling reliable and consistent results. SynThink Research Chemicals is a leading provider of high-quality CRMs, offering a wide range of products and services to support analytical testing needs.
In today’s fast-paced and quality-driven world, accurate analytical testing is paramount for industries such as pharmaceuticals. The use of CRMs has become increasingly important to ensure the accuracy, reliability, and comparability of measurement results. This article delves into the significance of CRMs in analytical testing and highlights the products and services provided by SynThink Research Chemicals.
Understanding Certified Reference Materials (CRMs)
Certified Reference Materials, also known as standards, are well-defined substances with precisely known compositions and properties. They undergo rigorous testing and certification processes to establish their accuracy, traceability, and stability. CRMs are used as a reference point for calibrating instruments, validating analytical methods, and ensuring the quality control of measurements.
Importance of CRMs in Analytical Testing
The use of CRMs is crucial for maintaining the accuracy, reliability, and comparability of analytical test results. They serve as a reliable reference to measure the performance of analytical instruments, detect and correct any bias or drift, and validate the accuracy of measurement methods. CRMs also contribute to the traceability of measurements, ensuring consistency and comparability across different laboratories and instruments.
SynThink Research Chemicals: Your Trusted Provider of CRMs
SynThink Research Chemicals is a reputable supplier of high-quality CRMs, catering to the diverse needs of the pharmaceutical industry. With a commitment to excellence, SynThink offers a wide range of products and services to support analytical testing and method validation.
Wide Range of Certified Reference Materials
SynThink Research Chemicals provides a comprehensive portfolio of CRMs, covering a wide range of substances and analytes. Their extensive catalog includes organic compounds, inorganic materials, impurities, metabolites, and more. These CRMs are meticulously characterized and certified to ensure their accuracy, stability, and traceability.
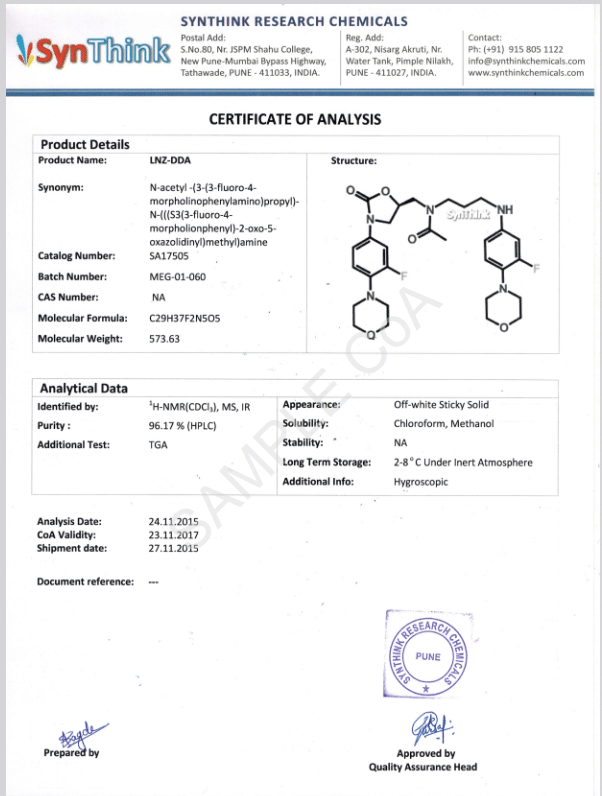
Custom Synthesis of CRMs
In addition to the standard CRMs available, SynThink Research Chemicals offers custom synthesis services. They understand that some analytical testing may require specific substances or impurities not readily available as CRMs. SynThink works closely with clients to synthesize custom CRMs that precisely match their requirements, providing tailored solutions for their analytical needs.
Characterization and Quality Control
At SynThink Research Chemicals, the characterization and quality control of CRMs are of utmost importance. Rigorous testing procedures are employed to verify the materials’ identity, purity, stability, and homogeneity. Advanced analytical techniques, such as chromatography, spectroscopy, and elemental analysis, are utilized to ensure the highest standards of quality.
Supporting Analytical Method Validation
CRMs are invaluable in the validation of analytical methods. SynThink Research Chemicals’ CRMs serve as ideal tools for assessing the accuracy, precision, linearity, and specificity of analytical methods. By incorporating CRMs into method validation studies, pharmaceutical companies can confidently establish the reliability and suitability of their analytical procedures.
Ensuring Regulatory Compliance
In the highly regulated pharmaceutical industry, adherence to regulatory guidelines is essential. SynThink Research Chemicals’ CRMs are certified according to internationally recognized standards and comply with regulatory requirements. By using these certified materials, pharmaceutical companies can demonstrate compliance, meet regulatory obligations, and ensure the safety and efficacy of their products.
Application of CRMs in Pharmaceutical Industry
CRMs find widespread application in various areas of the pharmaceutical industry. They are utilized in drug development, formulation, quality control, stability testing, impurity profiling, and bioanalytical assays. By employing CRMs, pharmaceutical companies can accurately quantify active pharmaceutical ingredients, detect and quantify impurities, and assess the performance of analytical instruments.
Benefits of Choosing SynThink Research Chemicals
When partnering with SynThink Research Chemicals for CRMs, pharmaceutical companies can benefit from:
- High-quality certified reference materials with accurate composition and properties.
- Custom synthesis services for tailored CRMs to meet specific testing needs.
- Rigorous characterization and quality control processes ensure the reliability of CRMs.
- Support in analytical method validation, facilitating compliance with regulatory requirements.
- Expert guidance and technical support from a trusted and experienced provider.
Certified Reference Materials are indispensable for accurate and reliable analytical testing in the pharmaceutical industry. SynThink Research Chemicals offers a comprehensive range of CRMs and services, ensuring the highest standards of accuracy, traceability, and quality control. By choosing SynThink as a partner, pharmaceutical companies can confidently meet regulatory requirements, enhance their analytical capabilities, and ensure the safety and efficacy of their products.
